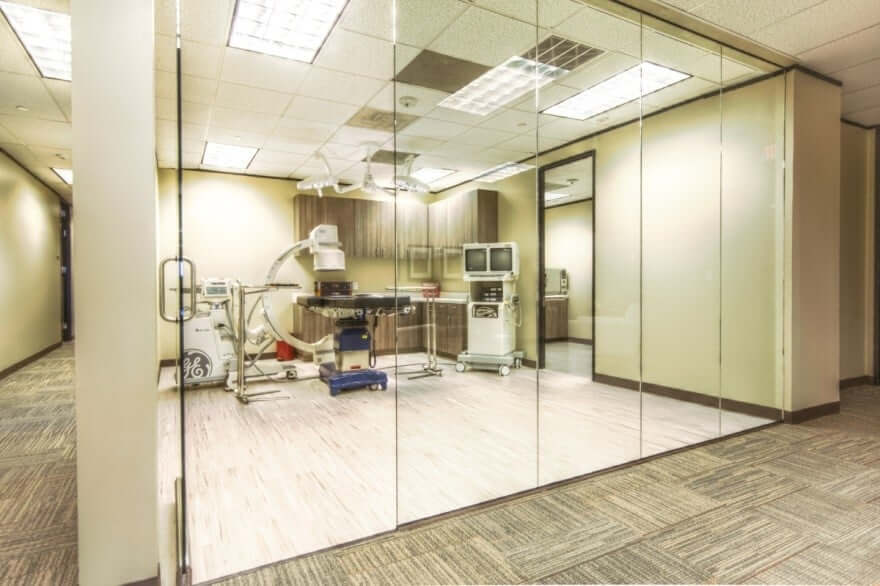
Genesys Spine Hosts First Cadaveric Labs in New On-site Facility
Austin, TX (March 30, 2016) – Genesys Spine announced the opening of its fully capable, on-site cadaveric lab. The lab features a fully functioning surgical suite equipped with an OEC 9800 C-Arm as well as sterile processing. This new facility allows Genesys Spine to conduct product demonstrations and training on their current product offerings as well as testing and training on future Genesys Spine products.
“We are excited to offer this new training and education facility to both existing as well as prospective customers. The ability to host these events on site allows us to provide both education and training on our growing portfolio of cutting-edge spine implants,” said Josh Kaufmann, principal, Genesys Spine.
The first cadaveric labs were held on February 19 and 26. The labs were focused on both the developmental testing of Genesys Products combined with demonstrations of several current products.
“Having recently participated in a cadaver lab at Genesys Spine’s headquarters, I was impressed by the first-class facilities. I believe that having a private cadaveric teaching lab such as this will definitely move Genesys to the next level within the spine industry,” said John Stokes, M.D., Seton Brain and Spine Institute.
About Genesys Spine
Founded in December of 2009, Genesys Spine was started by veterans of the spinal device industry. The founding partners have used their industry knowledge and input from existing surgeon relationships to introduce, into a mature spinal fusion market, an array of medical implants and instruments with novel characteristics. These devices, with their proprietary features, all remain within existing parameters for today’s current reimbursement codes.